ETHANOL
|
ETHANOL |
 Internal
combustion engines optimized for operation on alcohol fuels are 20 per cent more
energy-efficient than when operated on gasoline, and an engine designed
specifically to run on ethanol can be 30 per cent more efficient. Furthermore,
there are numerous environmental advantages, particularly with regard to lead,
CO2, SO2, particulates, hydrocarbons and CO emissions.
Internal
combustion engines optimized for operation on alcohol fuels are 20 per cent more
energy-efficient than when operated on gasoline, and an engine designed
specifically to run on ethanol can be 30 per cent more efficient. Furthermore,
there are numerous environmental advantages, particularly with regard to lead,
CO2, SO2, particulates, hydrocarbons and CO emissions.
Since ethanol has different chemical properties than
gasoline, it requires slightly different handling. For example, ethanol
changes from a liquid to a gas (evaporates) less readily than gasoline. This
means that in pure (100%) ethanol applications, cold starts can be a problem.
However, this issue can be resolved through engine design and fuel formulation.
Changes in engine design can also allow for greater efficiency. Although
one liter of ethanol has about two-thirds of the energy content of a liter of
gasoline, changes in engine design can allow for greater efficiency.
Furthermore, since ethanol is an organic product, should there be a spill, it
will biodegrade more quickly and easily than gasoline.
Ethanol as most important alcohol fuel can be produced
by converting the starch content of biomass feedstock (e.g. corn, potatoes,
beets, sugarcane, wheat) into alcohol. The fermentation process is essentially
the same process used to make alcoholic beverages. Here yeast and heat are used
to break down complex sugars into more simple sugars, creating ethanol. There is
a relatively new process to produce ethanol which utilizes the celluloses
portion of biomass feedstock like trees, grasses and agricultural wastes.
Cellulose is another form of carbohydrate and can be broken down into more
simple sugars. This process is relatively new and is not yet commercially
available, but potentially can use a much wider variety of abundant, inexpensive
feedstock.

Sugarcane residue, called bagasse - feedstock for ethanol.
Sugarcane is the world’s largest source for fermentation of ethanol. It is one of the most photosynthetic efficient plants - about 2,5 % photosynthetic efficiency on an annual basis under optimum agricultural conditions. A further advantage is that bagasse, a by-product of sugarcane production, can be used as the fuel for electricity production at some facilities. The tops and leaves of the cane plant can also be used for electricity production. An efficient ethanol distillery using sugarcane by-products can therefore be self-sufficient and also generate a surplus of electricity.
The production of ethanol by fermentation involves four
major steps:
(a) the growth, harvest and delivery of raw material to
an alcohol plant;
(b) the pre-treatment or conversion of the raw material
to a substrate suitable for fermentation to ethanol;
(c) fermentation of the substrate to alcohol, and
purification by distillation; and
(d) treatment of the fermentation residue to reduce
pollution and to recover by-products.
Fermentation technology and efficiency has improved rapidly in the past decade and is undergoing a chain of technical innovations aimed at using new alternative materials and reducing costs. Technological advances will have, however, less overall impact on market growth than the availability and costs of feedstock and the cost-competing liquid fuel options.
The many and varied raw materials for bioethanol production can be conveniently classified into three types:
(a) sugar from sugarcane, sugar beet and fruit, which may be converted to ethanol directly;
(b) starches from grain and root crops, which must first be hydrolysed to fermentable sugars by the action of enzymes; and
(c) cellulose from wood, agricultural wastes etc., which must be converted to sugars using either acid or enzymatic hydrolysis.
The last process is the new one and recently at the demonstration stage and still considered uneconomic. Of major interest are sugarcane, maize, wood, cassava and sorghum. Ethanol is also produced from lactose from waste whey; for example in Ireland to produce potable alcohol and also in New Zealand to produce fuel ethanol. A problem still to be overcome is seasonal production of crops, which means that quite often an alternative source must be found to keep a plant operating all-year round.


Ethanol production plant in Indiana (USA)
The biofuel gains differs for different feedstocks quite considerably (see the picture bellow). Sugar cane for ethanol production and palm oil for biodiesel seems to produce most fuel per hectare.

Ethanol and biodiesel yields from different feedstocks.
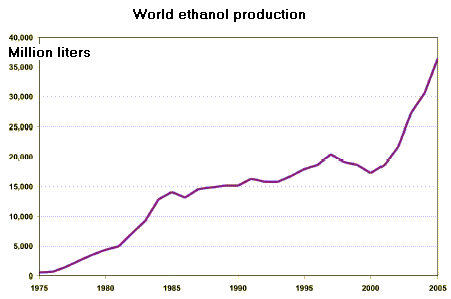
Currently, about 6 billion liters of ethanol are produced this way each year in the U.S. World-wide, fermentation capacity for fuel ethanol has increased eightfold since 1977 to about 20 billion liters per year. Latin America, dominated by Brazil, is the world’s largest production region of ethanol. Countries such as Brazil and Argentina already produce large amounts, and there are many other countries such as Bolivia, Costa Rica, Honduras and Paraguay, among others, which are seriously considering the ethanol option. Alcohol fuels have also been aggressively pursued in a number of African countries currently producing sugar - Kenya, Malawi, South Africa and Zimbabwe. Others with great potential include Mauritius, Swaziland and Zambia. Some countries have modernized sugar industry and have low production costs. The major objectives of large scale production of ethanol are: diversification of the sugarcane industry, reduction of energy imports, better resource use, and, indirectly, better environmental management. These conditions, combined with relatively low total demand for liquid transport fuels, make ethanol fuel attractive in developing countries. Global interest in ethanol fuels has increased considerably over the last few years due to the sharp increase of oil prices. Another reasons are increasing environmental concern, and also the possibility of solving some wider socio-economic problems, such as agricultural land use and food surpluses. In developing countries interest in alcohol fuels has been mainly due to low sugar prices in the international market, and also for strategic reasons. As the value of ethanol is increasingly being recognized, more and more policies to support development and implementation of ethanol as a fuel are being introduced.

GLOBAL ETHANOL PRODUCTION
Top Fuel Ethanol Producers in 2005.
| Production (million liters) | |
| Brazil | 16 500 |
| United States | 16 230 |
| China | 2 000 |
| European Union | 950 |
| India | 300 |
Production costs of ethanol and gasoline (2006).
| EUR per liter of gasoline equivalent | |
| Ethanol from sugar cane (Brazil) | 0,20 - 0 30 |
| Ethanol from corn (USA) | 0,30 - 0,50 |
| Gasoline (wholesale) | 0,30 - 0,55 |
| Ethanol from grain (EU) | 0,40 - 0,65 |
| Ethanol from cellulose | 0,65 - 1,00 |

Using ethanol even in low-level blends (e.g. E10 - which is 10% ethanol, 90% gasoline) can have environmental benefits. Tests have shown that E10 produces less carbon monoxide (CO), sulphur dioxide (SO2) and carbon dioxide (CO2) than reformulated gasoline (RFG). However E10 produces more volatile organic compounds (VOC), particulates, and nitrogen oxide (NOx) emissions than RFG. Higher blends (E85, which is 15% gasoline), or even pure ethanol-E100 - burn with less of virtually all the pollutants mentioned above.
Energy balance (the energy output:input ratio) is different for different feedstock. According the US data the production of ethanol is energy efficient as it yields almost 25 percent more energy than is used in growing the corn, harvesting it, and distilling it into ethanol. The most recent findings (2006) show that corn ethanol fuel is energy efficient and yields an energy output:input ratio of 1,5-1,6. Better results can be achieved with ethanol from sugar cane or celluloses ethanol (see the table below).
| Feedstock | Output : Input ratio | Reference |
| Celluloses ethanol | 2 - 36 | 2.62 Lorenz and Morris
(5+) DOE (10.31) Wang (35.7) Elsayed et al. |
|
Ethanol (sugar cane) |
2 - 8 | (2.09) Gehua et al.
(8.3) Macedo et al. |
|
Ethanol (wheat) |
2 - 4 | (2.05) ADEME/DIREM
(2.02–2.31) Elsayad et al. (2.81–4.25) Gehua |
|
Ethanol (sugar beets) |
2 | (1.85–2.21) Elsayad et al.
(2.05) ADEME/DIREM |
|
Ethanol (corn) |
1,5 |
(1.34) Shapouri 1995 (1.38) Wang 2005 (1.38) Lorenz and Morris (1.3–1.8); Richards |
Brazil
Since it was first introduced in 1975, the Brazilian Ethanol Program (ProAlcool)
remains to date the largest commercial application of biomass for energy
production and use in the world. It succeeded in demonstrating the technical
feasibility of large-scale ethanol production from sugarcane and its use to fuel
car engines.
Brazil first used ethanol as a transport fuel in 1903, and now has the world’s largest ethanol program in the world. Since the creation of the National ProAlcool Program in 1975, Brazil has produced over 90 billion liters of ethanol from sugarcane. The installed capacity in 2005 was over 16 billion liters. Over 16 billion liters of ethanol replaced about 250,000 barrels of imported oil a day.
In 1979 there was almost 5 million automobiles which run on pure ethanol
and a further 9 million running on a 20 to 22 per cent blend of alcohol and
gasoline. From 1976 to 1987 the total investment in ProAlcool reached 7 million
USD and the total savings so far for Brazilian economy reached USD 400 billion
in imports. In 1988 almost 100% of new cars were ethanol powered. After the
decline of oil price (bellow 20 USD/barrel) the ethanol program in Brazil
declined considerably (there were almost no new ethanol cars sold in 1997 and
1998. With “high”oil prices over 40 USD/barrel (since 2005) there was
another economic incentive for ethanol program expansion. Today Brazil
operates almost 50% of their vehicles on pure ethanol with the rest running on
blends. A 10% blend requires no engine modifications at all.
Apart from ProAlcool’s main objective of reducing oil
imports, other objectives of the program were:
Although ProAlcool was planned centrally, alcohol is produced entirely by
the private sector in a decentralized manner. The ProAlcool program has
accelerated the pace of technological development and reduced costs within
agriculture and other industries. Brazil has developed a modem and efficient
agribusiness capable of competing with any of its counterparts abroad. The
alcohol industry is now among Brazil’s largest industrial sectors, and
Brazilian firms export alcohol technology to many countries. Another industry
which has expanded greatly due to the creation of ProAlcool is the ethanol
chemistry sector.
Ethanol-based chemical plants are more suitable for many developing countries
than petrochemical plants because they are smaller in scale, require less
investment, can be set up in agricultural areas, and use raw materials which can
be produced locally.
SOCIAL DEVELOPMENT
Rural job creation has been credited as a major benefit of ProAlcool because
alcohol production in Brazil is highly labor-intensive. Some 700,000 direct jobs
with perhaps three to four times this number of indirect jobs have been created.
The investment to generate one job in the ethanol industry varies between 12,000
USD and 22,000 USD, about 20 times less than in the chemical industry for
example.